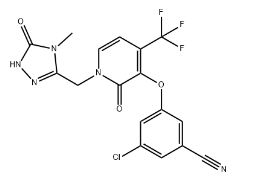
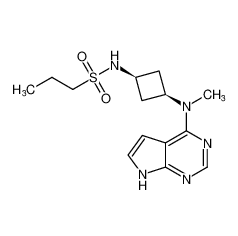
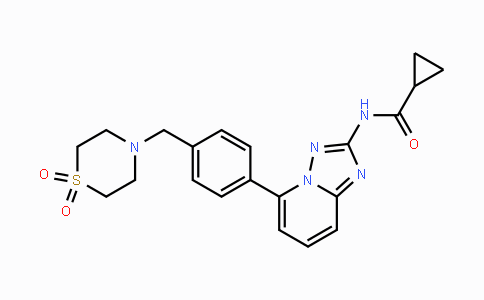
Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced that the U.S.
READ MORE 2020-12-22News List
Merck announced recentlythat its new HIV drug Pedro (doravirin tablets) has been officially approved
READ MORE 2020-12-08Pfizer announced that the U.S. Food and Drug Administration (FDA) accepted for filing and granted Pr
READ MORE 2020-11-02ViiV Healthcare, the global specialist HIV company majority owned by GSK, with Pfizer Inc. and Shion
READ MORE 2020-10-18Gilead Sciences and Galapagos NV today announced that the European Commission (EC) has granted marke
READ MORE 2020-09-27The Janssen Pharmaceutical Companies of Johnson & Johnson announced today that the European Comm
READ MORE 2020-09-09Approval is based on results from SOLAR-1, where PIQRAY®in combination with fulvestrant nearly doubl
READ MORE 2020-08-20Blueprint Medicines announced that the European Medicines Agency (EMA) Committee for Medicinal Produ
READ MORE 2020-07-29At present,The COVID-19 epidemic continues to spread rapidly around the world. As of 01am, July 04,
READ MORE 2020-07-05Dear Customers,Unfortunately, we have to cancel the plan for our participation in the CPHI North Ame
READ MORE 2020-06-18