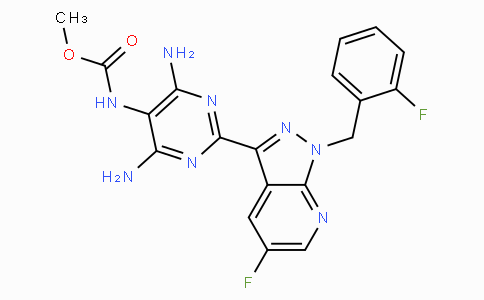
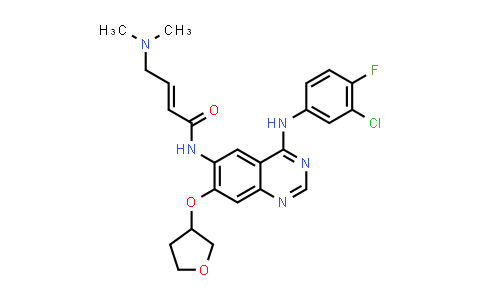
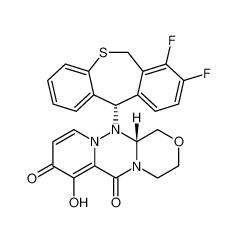
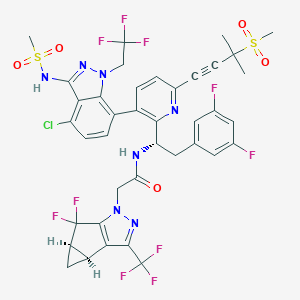
August 17, 2021 – Novartis today announced that the China National Medical Products Administration (
READ MORE 2021-08-22News List
Berlin, Germany, July 21, 2021– The European Commission has granted marketing authorization in the E
READ MORE 2021-07-26CStone's partner Blueprint Medicines recently announced that the U.S. Food and Drug Administrati
READ MORE 2021-06-24Late-breaking data analyses presented by AbbVie (NYSE: ABBV) at Digestive Disease Week®(DDW) Virtual
READ MORE 2021-06-01On April 29, 2021, Roche China announced that its innovative influenza drug Xofluza® (Mabaloxavir) h
READ MORE 2021-05-13AstraZeneca'sTagrisso(osimertinib) has been approved in China for the adjuvant treatment of pati
READ MORE 2021-04-15Gilead Sciences, Inc. (Nasdaq: GILD) today presented additional results from the Phase 2/3 CAPELLA t
READ MORE 2021-03-15On June 18, 2020, BeiGene, Ltd. (“BeiGene” or the “Company”), a commercial-stage biotechnology compa
READ MORE 2021-02-25January 7, VISEN Pharmaceuticals announced that it had submitted TransCon CNP (TransCon C-type natri
READ MORE 2021-02-08Recently, Wang Junfeng's research team from Hefei Institute of Material Science, Chinese Academy
READ MORE 2021-01-19