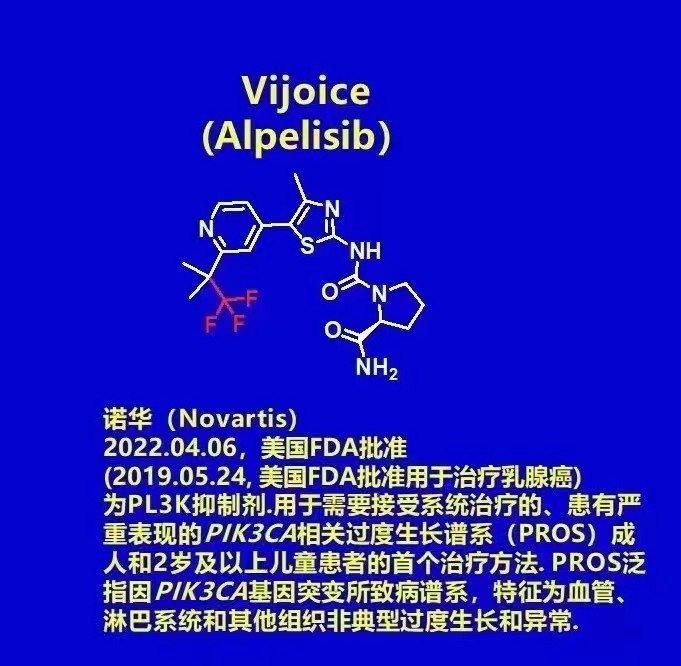
2022.04.06, Novartis announced that the U.S. Food and Drug Administration (FDA) has granted accelera
READ MORE 2022-07-06News List
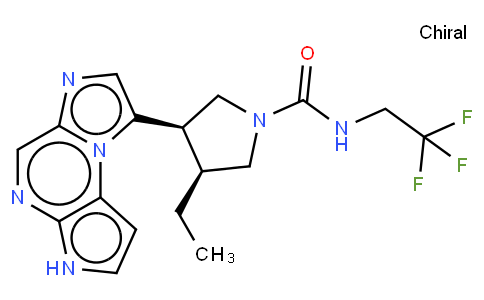
Bristol-Myers Squibb (BMS) recently announced positive results from the Phase 2 PAISLEY study (NCT03
READ MORE 2022-06-13Takeda announced the Phase 3 SHP643 to evaluate the safety and pharmacokinetics of Takhzyro (lanadel
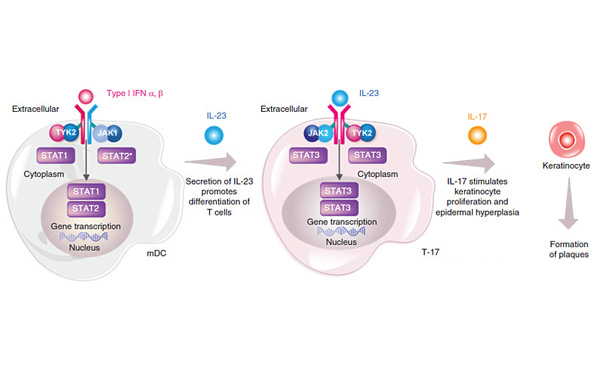
READ MORE 2022-05-13Recently, Bristol-Myers Squibb (BMS) presented 2-year data from the POETYK PSO long-term extension t
READ MORE 2022-05-12AbbVie recently announced that China’s National Medical Products Administration (NMPA) has approved
READ MORE 2022-03-11Genentech, a member of the Roche Group, today announced that the U.S. Food and Drug Administration (
READ MORE 2022-02-10ViiV Healthcare announced that the US Food and Drug Administration (FDA) approvedApretude, the first
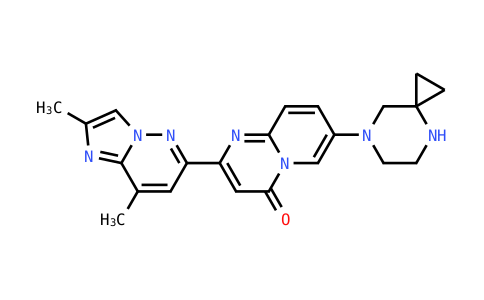
READ MORE 2021-12-22Novartis’ asciminib hasoutperformedPfizer’s Bosulif in a phase 3 chronic myeloid leukemia (CML) stud
READ MORE 2021-11-01On October 11, 2021, Jiangsu Hengrui Pharmaceuticals Co., Ltd. announced that SHR0302, for the treat
READ MORE 2021-10-15Merck (NYSE: MRK), known as MSD outside the United States and Canada, announced today that the U.S.
READ MORE 2021-09-07