The world's second! FDA grants Pfizer priority review for CD3/BCMA bispecies
2023-02-23
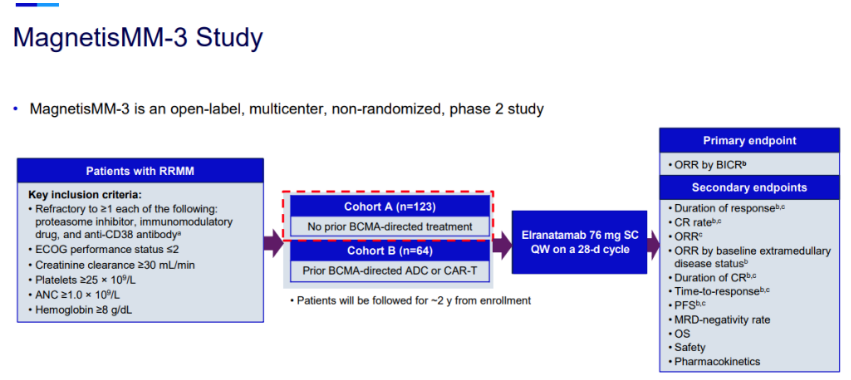
Pfizer announced that the U.S. Food and Drug Administration (FDA) has granted priority review status for its Biologics License Application (BLA) for the CD3/BCMA bispecial antibody elranatamab, with an FDA decision expected in 2023. It also indicates that elranatamab is expected to become the second approved CD3/BCMA bispecial antibody in the world, following Johnson & Johnson /Genmab's teclistamab. Meanwhile, the European Medicines Agency (EMA) has accepted the Marketing authorisation application (MAA) for elranatamab.
